General introduction
Living nature can be divided into different areas with specific plants and animals. The group of organisms in a certain area and the way in which these organisms interact with each other is called an ecosystem. Examples of ecosystems are coral reefs, deserts, taigas, rain forests, and savannas. The outward boundaries of ecosystems are set by physical factors such as temperature, rain, and the geological shape of the land. Polar regions are different from the tropics in temperature, but within the tropical zone, there are wet and arid areas. The geological shape can mean plains or mountains, small islands or large continents, shallow or deep sea. Organisms are adapted to live under specific circumstances and the occurrence of such circumstances determines which animals and plants can be found where. These influences are called abiotic factors. Relations between the organisms determine the detailed composition of that ecosystem (e. g. predators eating prey, trees creating a place for birds to build a nest, corals and sponges competing for space on a reef flat, etc.). Such influences of organisms on each other are called biotic factors. The availability of nutrients depends on a combination of abiotic and biotic factors.
The term ‘nutrient’ in a broad and general sense means food. Organisms need nutrition or food to obtain the necessary energy and building materials to grow, maintain and reproduce. However, more commonly the term nutrients is used for the chemical elements nitrogen and phosphorus. With nutrient pollution or eutrophication we mean an increase in nitrogen (usually as ammonium or nitrate) and phosphorus (as phosphate) in a natural environment. Before I go into the details of eutrophication, let me first explain the role of the elements in an ecosystem.
Plants fix energy from sunlight into organic material in a process called photosynthesis. Plant-eating animals (herbivores) obtain the necessary energy to live by eating plants. Animal-eating animals (carnivores) eat herbivores or other carnivores. This way energy is transferred through the food chain from plants to herbivores to carnivores. It is important to realize that there is only one input in the system: plants fixing sunlight. All other organisms depend on the presence of plants for their energy. Energy is transferred through the ecosystem until it is lost.
Aside from fixing energy into organic material, a plant needs building materials to make itself: stem, leaves, roots, flowers; the whole thing. These materials are usually expressed as their chemical elements, e.g. carbon (C), nitrogen (N), phosphorus (P), hydrogen (H), oxygen (O), etc. In reality, these elements are bound in organic molecules. C, O, and H form the largest part of living or organic matter. Nitrogen is a necessary element in for example protein molecules and phosphate occurs in cell membranes. Also, both elements N and P are necessary parts of DNA. Other elements are needed in small amounts to form a body, such as iron and copper. In a whole living body, these materials are needed in certain amounts. Plants in the sea consist of C and N and P in a ratio of approximately 106:45:5. Plants need to obtain these different elements in different amounts from the environment. C is present in CO2, H is in water (H2O), N in NH4 (ammonium) or NO3 (nitrate) and P in PO4 (phosphate). O is present in almost all these molecules. The ratio in which these building materials are available is mostly not the same as the ratio in which they are needed. Of one of these elements, there will be less available relative to the others, which means that this element becomes limiting for growth. In the sea, there is of course water enough and H and O are never a problem. CO2 dissolves into the water from the atmosphere and is usually sufficiently present as well. The limiting nutrient is most commonly N or P (although there are areas where neither N nor P, but iron is limiting). Hence the common use of the term nutrient pollution for excess inputs of ammonium (NH4), nitrate (NO3), and phosphate (PO4).
Like energy, nutrients are transferred through the ecosystem as one organism eats another. There is, however, an important difference: nutrients are not used up but become released again. Animals that eat plants burn 80 – 90% of their food for energy and use only the rest for the growth of their body and reproduction. This means that they eat far more N and P than they need and the surplus has to be excreted. Also, every organism dies at some time and when bacteria break down the remains, nutrients become available again. The essential difference with energy is that nutrients are cycled through an ecosystem. Plants take up inorganic nutrients from their environment and fix them in organic material, animals eat plants and excrete organic nutrients, and bacteria convert these back to inorganic nutrients, which can be used by plants again. As long as none are lost, nutrients could in theory be recycled forever through an ecosystem. In reality, ecosystems are not closed and nutrients are imported and exported: animals move in or away, water currents bring or take away organisms and molecules, dead organisms disappear into deep water, etc. In long-living ecosystems the import and export of nutrients are usually balanced: as much comes as goes out.
One of the major effects of humans on their environment is that we change the nutrient balance by increasing the nutrients concentrations. We use fertilizers in agriculture, which is nothing else than nutrients for those plants we wish to grow. These plants cannot use all the nutrients we supply and much of the loading is lost to the environment. Sewage consists of nutrients in organic and if treated in a sewage plant, inorganic forms. These nutrients are generally discharged into our environment. At the same time, we often reduce the capacity of the nature around us to use these nutrients by removing the natural vegetation for agriculture or urban development. Humans eutrophy their environment and the larger and denser the population is, the stronger the nutrient pollution.
So, why is this a problem? We are basically giving plants and thereby all the animals in the ecosystem materials that they need, don’t we? The answer is that reality is not that simple. Yes, plants need nutrients, but only a limited amount. The problem is that increases in nutrients lead to changes in the ecosystem. Some plants are specialized to survive in an environment with low nutrient concentrations, while other plants dominate with high nutrient concentrations. When nutrient levels are increased the ecosystem shifts from low nutrient specialists to high nutrient specialists. Ultimately this leads to completely different ecosystems under long-term eutrophication. Generally, this leads to a reduction of the diversity within ecosystems and variation between ecosystems.
The coral reef ecosystem.
Coral reefs consist of many different organisms: macro-algae, stony corals, soft corals, sponges, ascidians, snails, mussels, crabs, lobsters, fish, etc. Macro-algae are plants such as seaweeds, coralline algae, small turf algae, etc. The other groups are basically animals, but there are a few important strangers: animals that have unicellular plants living in their skin. These algae are called zooxanthellae or in short zoom. These combinations (or symbionts) behave partly as plants and partly as animals. Most well-known are stony corals, but other examples are some soft corals, some sponges, giant clams, and the upside-down jellyfish (Cassiopeia). The zooxanthellae fix the energy of sunlight by photosynthesis and the animal catches food from the water column (small bacteria, algae, and animals). Because of the dependence on sunlight corals can only live in clear and shallow waters. The zoox gives energy to the coral (or another host) and the coral gives nutrients in return. By living together in a symbiosis both organisms do better than if they were living apart. The other animals without zooxanthellae make a living by catching food particles from the water column or by eating other reef organisms.
Stony corals make and shape the coral reef. They contribute most to reef-building by the limestone skeletons they make to grow. The collection of stony corals in a reef creates the three-dimensional structure of the reef. There are many gaps and crevices between and under coral colonies, which serve as hiding places for many other organisms to survive in a coral reef. Where corals disappear many other creatures such as lobsters and colorful fish are lost as well. Besides corals, crustose coralline algae also contribute to reef growth by calcification. At the same time that a coral reef is built by corals, it is also broken down again by bio-erosion. Parrotfish eat small algae growing on dead coral but in the process of eating grind the old coral skeleton to dust or sand. Boring sponges and mussels drill holes in coral skeletons. The balance of reef growth and destruction determines whether a reef as whole increases or decreases. This balance depends on how many of which organisms are present in a reef ecosystem.
Coral reefs occur typically in waters with low nutrient concentrations. Reef organisms are adapted to survive under these low nutrient concentrations. Corals and macro-algae can take up inorganic nutrients directly from the surrounding water although these occur in very low concentrations. Organic nutrients are gained in food collected from the water column. As the surrounding oceanic water flows over the reef many nutrients are subtracted from this water. Another, and possibly very important, input of nitrogen is the fixation of atmospheric N2 into amino acids. Nutrients are also lost to the overlying water column and taken away with the current. Nutrients are not contained cycle after cycle in the reef ecosystem, but rather taken up by the plants and animal-plant symbionts and lost again when excreted by higher trophic levels. Nutrients flow through the food chain and are converted from one form to another in the process. Aside from the classic food chain described above, bacteria are responsible for many transformations of nutrients. Bacteria can for example convert ammonium to nitrite to nitrate. All in all the reef ecosystem is an extensive, complex network of compartments that do different things with different nutrients.
Eutrophication effects in coral reefs.
Increases in nutrient concentrations have various effects on the coral reef ecosystem. The first set of problems occurs on the level of individual organisms. In corals the zoox—coral symbiosis becomes disturbed with high nutrient concentrations. When corals are kept in aquariums with high ammonium concentrations for a few weeks, the zoox multiplies strongly and coral growth stops. It is not known yet what exactly happens, but it is quite clear that the zoox use the energy from photosynthesis to grow themselves instead of giving it to the coral. Elevated nutrient concentrations are bad news for corals. On the level of primary producers (plants), the competition between corals and macro-algae is influenced by nutrient concentrations. Since the bottom surface area on a reef is limited (1 m2 is 1 m2) reef organisms have to compete for space. They all need a hard bottom to attach themselves to and cannot grow on top of each other. With low nutrient concentrations, corals are able to keep algae away and overgrow them. With high nutrient levels, algae get a competitive advantage and start to overgrow corals. As algae do not calcify, reef growth is reduced. An important factor influencing coral vs. macro-algae competition is fisheries. Many fish, such as parrotfish, eat macro-algae. When these fish are removed, the control on macro-algal growth is removed, which is again bad for corals. On a more complex ecosystem level, eutrophication can lead to an increase in bacteria, phytoplankton, and their consumers in the water column. More particles become available in the water column and bio-eroders, such as boring sponges and mussels can use this extra food. This leads to more drilling of holes in the coral skeleton, which weakens the corals. If their skeleton is not strong, they easily break off during storms. Again bad news for corals.
There are various well-known examples of the effects of eutrophication on coral reefs in the scientific literature. On Barbados, reduced growth, reduced reproduction, reduction of successful settlement, and changes in the coral composition have been recorded. In Jamaica the coral reef has been replaced by a macro-algae or seaweed reef helped strongly by heavy overfishing and disease that wiped out the sea urchins. In Kaneohe Bay, Hawaii, heavy sewage discharge led to enormous amounts of macro-algae (up to 2.5 m high!) and an almost complete loss of corals. After the sewage discharge was diverted to deep water away from the island, the macro-algae disappeared and corals have come back to a reasonable extent. Although the precise causative mechanisms are often still unclear, there is no doubt that eutrophication has serious negative effects on the health of coral reefs. —
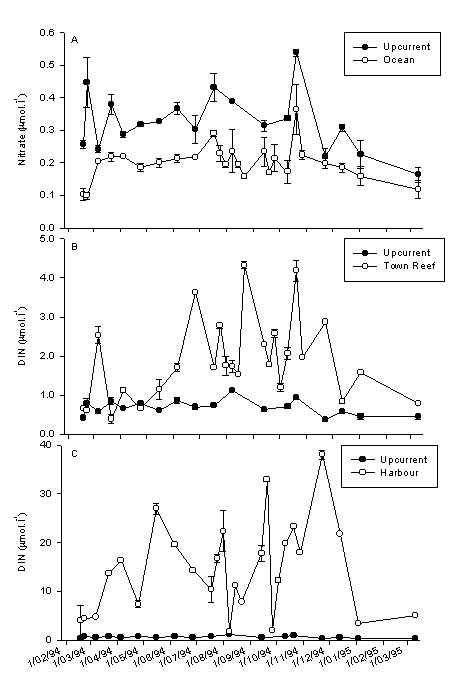
Figure 1. Inorganic nitrogen concentrations at 2 m depth in coral reef and the adjacent oceanic water in Curaçao. A: Oceanic water compared to non-eutrophied reef water. B: Eutrophied compared to non-eutrophied reef water. C: Harbor water. DIN = Dissolved Inorganic Nitrogen (= NH4+ + NO2– + NO3–). Mean ± sd (n=4).
Eutrophication in Curaçao
On Curaçao, there are three main sources of nutrient pollution. The first and most obvious is sewage discharge along Willemstad. At Marie Pompoen, beside the Avila Beach Hotel and near the old library in Punda there are 3 sewage pipes that discharge about 1000 m3 of untreated sewage per day. A lot of sewage is also discharged into the Waaigat, Schottegat, and Anna Bay (there is a report that indicates the amounts by Letitia Buth and Tico Ras). Also, industrial waste from the refinery, the slaughterhouse, etc. is discharged into the Schottegat. With each outgoing tide and after heavy rain the water flows out of the Anna Bay and over the reef east or west of it. A second, more erratic, source of mainly inorganic nutrients is runoff with rain. Rainwater runs off the streets and through drainage channels to the sea and takes rubbish, sediment, etc. from the land to the water. The third and least visible source of nutrients is groundwater seepage. Only 38% of the households on Curaçao are connected to the sewage system and the rest uses septic tanks. In septic tanks, organic matter is broken down by bacteria, which changes nutrients to inorganic forms, but does not remove them. Water from the septic tanks sinks down into the bottom. Most of the bottom under Willemstad consists of fossil reefs, which are very porous and the water easily seeps through. On average, the total of septic tank water is equal to the total of rainwater that seeps through to the groundwater. This results in very high nitrate concentrations in the groundwater. At the same time, some groundwater is pumped up and used for irrigation, but this happens mainly further away from the town. When more and more septic tank and rainwater is added to the groundwater, this is eventually pushed sideways out of the ground and onto the reef. It will depend strongly on the amount of rain in a year or season how much comes out, but come out it does.
Which of these influences can be recognized in patterns of nutrient concentrations at Curaçao? I sampled water at different stations along the coast of Curaçao and measured inorganic nutrients from February 1994 until March 1995 with 2 or 3-week intervals (see map on page 4)
Station Ocean is 3 km away from the island in deep oceanic water.
Station Upcurrent is just east of Fuik, where clean oceanic water comes in and flows over the reef. There is no urban development or agriculture at Eastpoint and hence no influence of humans.
- Station 2 is in front of Jan Thiel Lagun.
- Town Reef is just west of the Avila Beach Hotel in front of Punda.
- Harbour is in the mouth of Anna Bay under the floating bridge.
- Station 5 is between Sonesta and Caribbean hotels.
- Station 7 is Slangenbaai
- Downcurrent is Pestbaai (a.k.a Vrijgezellenbaai).
In Fig. 1A comparison of nitrate concentrations is made between non-eutrophied reef water and the adjacent ocean. The nitrate concentration was always higher in the water above the reef. The ammonium concentration was the same in the reef and oceanic water (the same concentrations as nitrate). Only nitrate was enhanced. Since there are no influences of humans at this reef such as sewage and groundwater seepage, this nitrate must have been excreted by reef organisms or micro-organisms in the sediment. This is a natural phenomenon in reef waters and has previously been found on other reefs, e.g. in the Great Barrier Reef. There was no difference in phosphate concentrations at Fuik and Ocean (Fig. 2).
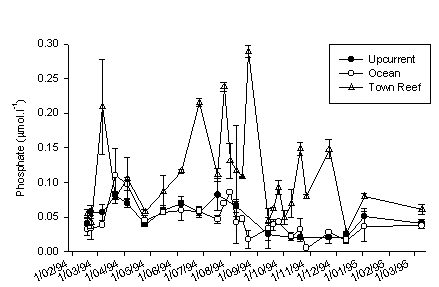
Figure 2. Phosphate concentration at 2 m depth in eutrophied and non-eutrophied reef waters and the adjacent oceanic water. Mean ± sd (n=4).
Fig. 1B shows a comparison of eutrophied and non-eutrophied reefs in DIN (Dissolved Inorganic Nitrogen, which is ammonium + nitrite + nitrate; nitrite is less than 1 % of DIN). On most of the days I sampled, DIN levels were strongly elevated in front of town. There is large variation through time because sewage discharge is not continuous and the water current over the reef flat is highly variable. Note that the scale of Fig 1 B is ten times larger than that of 1A. DIN at Town Reef consists roughly of 50% ammonium and 50% nitrate. Phosphate concentrations were also usually higher at Avila than at Fuik or in the adjacent ocean (Fig. 2). From July to October 1994, weekly samples were collected and more reef stations were included. The results are shown in figure 3, where the open circles indicate Ocean, the filled diamonds Avila, and the bars various reef sites. Different patterns become clear in this figure. In week 6 (22 Aug.) both ammonium and phosphate peaked. Smaller peaks of these two nutrients occurred in week 2. This is a general phenomenon that can also be seen in figures 1B and 2: ammonium and phosphate are both high or both low. The reason is that sewage is collected in large underground reservoirs which are emptied when full. This results in an erratic discharge pattern. On some days I sample in a sewage cloud, but on other days I caught normal reef water. The latter does not mean that there was no sewage discharge, but merely that I missed it. Another pattern that emerges from figure 3 is that nitrate behaves very differently from ammonium. Nitrate was not elevated in week 6, but clearly so in weeks 9, 10, and 11. This shows that the source of nitrate cannot be sewage (the reason no nitrate comes out with sewage is that bacterial activity in the underground reservoirs depletes oxygen, which prevents oxidation of NH4 to NO2 to NO3). This nitrate came into the reef by groundwater seepage. There was rain at the end of August and some groundwater with high nitrate concentrations gradually seeped out during the following weeks. We can conclude that there is nutrient pollution in the reef water column in front of Punda caused by sewage discharge and groundwater seepage.
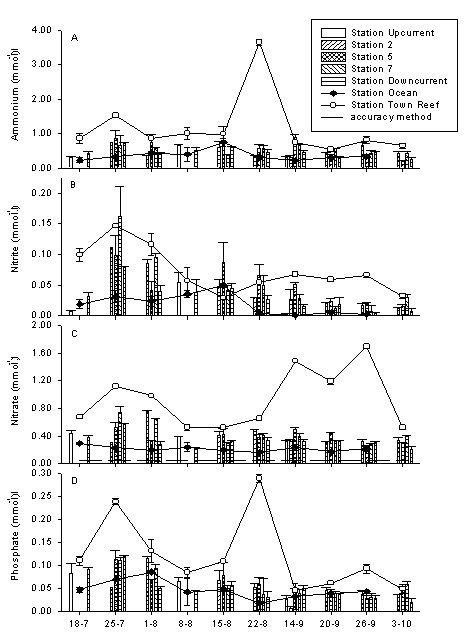
Figure 3. Nutrient concentrations at 2 m depth in fringing coral reef waters and adjacent ocean in Curaçao. Stations along the southern shore. Town reef is eutrophied, bars indicate sites away from eutrophication. Mean ± sd (n=4). Accuracy is accuracy of the measurement methods.
DIN levels in the Anna Bay are shown in figure 1C. Note that the scale is again enlarged by almost a factor 10. This DIN consists for about 80% of ammonium. Large fluctuation is caused by the tide. With in going tide I sampled reef water flowing in and with ebbing tide I sampled increasingly more polluted water from further down the bay. These values are extremely high. The reason is that severe eutrophication is combined with a very long residence time of the water. Sources of eutrophication are sewage, runoff and groundwater seepage (indicated in this case by silicate levels; silicate originates from the volcanic core of the island). The water exchange time of the Anna Bay and Schottegat is probably in the order of magnitude of 100 days (it must be noted that this figure depends strongly on the amount of rain). It takes a very long time before nutrients are washed out of the bay. Phosphate concentrations in the Harbour are comparable to those at Avila. This shows that groundwater seepage is relatively more important, because phosphate is bound to limestone in the old reefs. An important question is how far the pollution from the Harbour reaches along the shore. As shown in Fig. 4 elevated ammonium, nitrite and nitrate can be measured up to 4 km down current of the harbour with outgoing tide. This number should be used very carefully, as I did this long transect on one day only. When there are high waves and a strong current, the pollution will be diluted very rapidly, but on calm days the harbour water may reach much further along the coast.
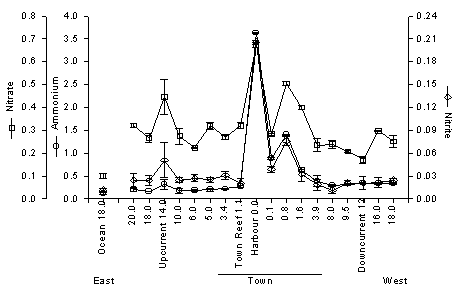
Figure 4. Dissolved inorganic forms of nitrogen in coral reef waters and adjacent oceanic water along the southern coast of Curaçao on 22 February 1994, with outgoing tide. Numbers on the x-axis indicate distance in km to the harbour in the middle of town. Town ranges from 4.5 km east to 4 km west of Harbour. Mean ± sd (n=4).
State of the coral reefs along the southern shore of Curaçao.
Healthy, well developed reefs with many species and large colonies can be seen near Eastpoint where clean oceanic water arrives at the island and no eutrophication occurs. Aside from a few spots with local problems (Fuik, Caracas Bay) reefs are still in rather decent shape up to Seaquarium where the construction of the Seaquarium breakwaters and beach have completely exterminated the coral reefs. Between Seaquarium beach and Princess Beach there is a drainage channel which is usually dry, but with heavy rain much sediment and organic nutrients come out causing very turbid waters. Between this channel and the Anna Bay there are 3 sewage pipes and some artificial beaches. The reefs are strongly degraded over this stretch. I have measured in 1991 that both total coral cover and the number of species are reduced by 50% at Avila compared to the reef east of Seaquarium. Acropora palmata and A. cervicornis and Porites porites are completely gone, the Agaricia’s have mostly disappeared and only head corals survive (Diploria’s, Montastrea’s, Colphophyllia natans, Siderastrea siderea, Porites asteroides) and Madracis mirabilis manage to hold. These findings have more recently been repeated by students of Prof. Rolf Bak at Marie Pompoen and Avila. They also showed that especially the baby corals are missing. This is very worrisome, because new recruitment is needed to get restoration of the reefs. Another important finding has been by Erik Meesters and students who have shown that more injuries occur on corals in front of the town and that these heal slower. West of the Anna Bay the reef terrace is bare coral rock for the first kilometer or so. There is nothing that survives the mixture of high nutrients, metals, oil and other toxic chemicals that come out of the harbor bay. In front of Holiday Beach there are some poorly developed corals again that try to make a living. The coral reef basically isn’t much until a few kilometers past the Piscadera Bay. In general, the coral reefs in front of Willemstad are strongly degraded which is related to the presence of that town.
It is impossible to determine exactly how much of this degradation has been caused by nutrient pollution. Other important direct or indirect destructive factors are or could be: sedimentation (both from runoff and artificial beaches), overfishing, toxic chemicals in sewage (what are effects of chloride and detergents on corals?) and oil pollution. Moreover such negative influences often enhance each other. However, there is no doubt that nutrient pollution is a serious problem afflicting the health of the coral reefs along Curaçao and that counter measures should be taken.
The simplest and cheapest “end of the pipe solution” to nutrient pollution is to literally lay the end of the pipes further out in deeper water away from the reefs. This would certainly reduce the direct effects of sewage discharge on the coral reef of Curaçao and should be considered as a TEMPORARY first solution. On the long term this would not solve all the problems and lead to healthy coral reefs. First, this discharge still contributes to the general eutrophication of the ocean and may lead to negative effects on far longer time and spatial scales than we can currently see. Moreover, we have no idea what effects of chemicals in the sewage are and these may very well be pretty destructive at very low concentrations. Dilution is no solution to pollution. The only way to solve the eutrophication problem is to connect all households (no more septic tanks) and industries to a sewage system, treat that water and remove the nutrients. Although this is very expensive and will take considerable time to develop, it is the solution that should be worked towards. Using secondary treated sewage water for irrigation is a good alternative to removing the nutrients in a tertiary treatment step as long as these nutrients do not sink into the groundwater and seep out to the reef. Second, as long as the harbour stays as heavily polluted as it is at the moment, it will remain a source of nutrients and other toxic chemicals. It is really time that the industry around Schottegat is forced to live up to modern environmental standards and clean up their rubbish. Third, runoff and sedimentation will stay serious problems that have to be dealt with. Basins with vegetation (the good old mangroves) should be constructed in which the sediment in runoff water can sink out before the water reaches the sea. Artificial beaches should not be allowed anymore. Every reef in front of an artificial beach is a desert.
Afterword
What Curaçao needs is a long term commitment and realistic plan to counter the effects of the dense population on its reefs and these intentions need to be enforced by laws. Healthy coral reefs along the southern coast of Curaçao are no utopian idea. If industries, dive operators, recreational users, environmental NGO’s, underwater park management and government services are willing to commit themselves and cooperate both among each other and with scientists and law enforcers, the coral reefs of Curaçao can be saved and improved. Essential, however, is that protection of the reefs is not placed second in priority to short term economical gains. It is realistic to state that if nothing is changed Curaçao will have no more coral reefs in a few decades. But not all is bleak and hopeless, construction of a sewage system for the Punda side of town is now finally underway while a sewage system for the Otrobanda side was completed a few years back. Things are changing and can change further. It is up to the people of Curaçao to choose the changes in the right direction, not only for the wellbeing of the corals and all the other reef organisms, but for themselves as well. Curaçao without coral reefs would be a sad development indeed.
Resource: laser eye surgery.
